BioPredictive's five main diagnostic tests are included in NASH-FibroTest, for a complete assessment of the condition of the liver and the five main causes of liver disease 1 .
NASH-FibroTest = FibroTest + SteatoTest 2 + NashTest 2 + ActiTest + AshTest
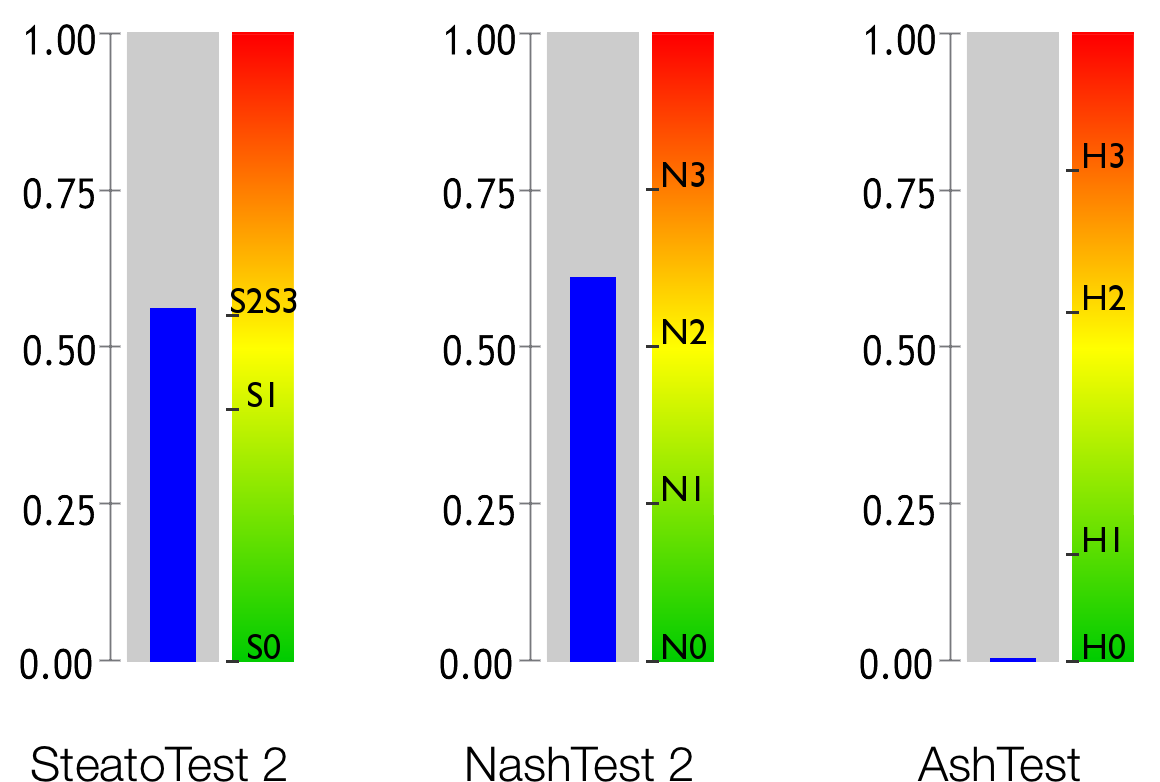
Hepatic steatosis, which is assessed by the SteatoTest 2, is a build-up of fat in the liver, which frequently causes elevated levels of Gamma-GT and transaminases.
Non-alcoholic steatohepatitis (NASH) is an inflammatory disease of the liver which is caused by metabolic conditions including excess weight, arterial hypertension (high blood pressure) and abnormal levels of triglycerides or cholesterol. The NashTest 2 evaluates the level of necroinflammatory activity caused by the metabolic condition.
Alcoholic steato-hepatitis (ASH) is an inflammatory disease of the liver caused by excessive alcohol consumption. The AshTest evaluates the level of this necroinflammatory activity due to alcohol.
Fibrosis and necroinflammatory activity are the two main causes of liver disease.
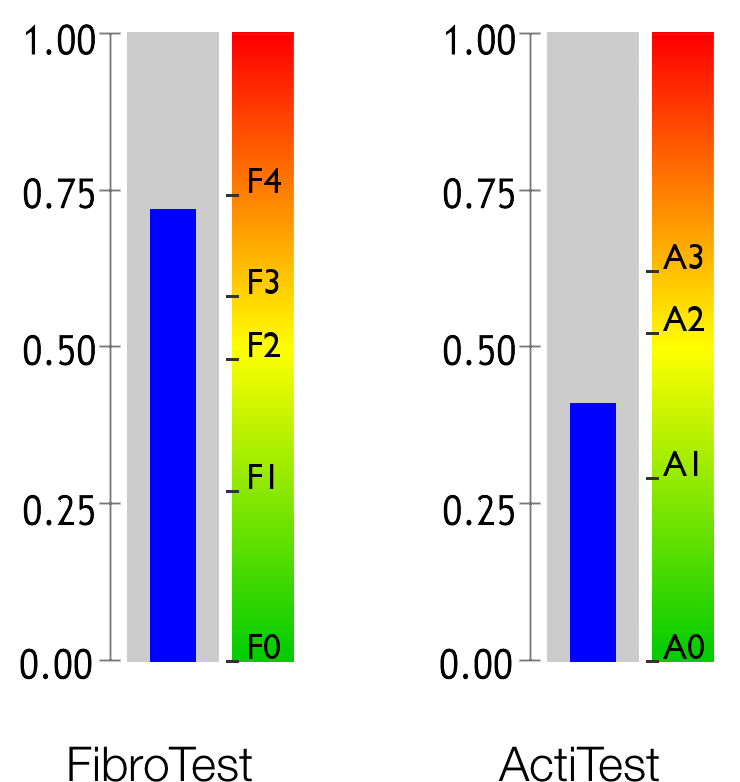
Fibrosis is a medical condition caused by the reaction of a diseased liver. Hepatic fibrosis is typically compared to a form of scar tissue that progresses throughout the liver. The most serious stage of fibrosis is known as cirrhosis.
Activity refers to the level of liver inflammation caused by disease. It is often compared to a burn.
FibroTest is recommended by WHO 2 , the american Association for the Study of Liver Diseases (AASLD) 3 , the European Association for the Study of the Liver (EASL) 4 , and the Asia-Pacific Association for the Study of the Liver (APASL) 5 for testing for hepatic fibrosis in chronic hepatitis C patients, with or without HIV co-infections, as well as patients with metabolic conditions or who consume excess alcohol. 6 7 8
FibroTest is used to give access to non-interferon treatments for combating the hepatitis C virus and for patient monitoring 9 .
FibroTest, when combined with ActiTest, makes it possible to identify asymptomatic carriers of the hepatitis B virus as well as potential treatments 10 .
FibroTest is specifically designed for cirrhosis and approved for the purposes of classifying its severity into one of three classes 11 .
FibroTest is the only test that is capable of classifying the early stages of fibrosis 12 .
FibroTest can be used for longitudinal monitoring of patients with chronic liver disease 9 13 14 .
ActiTest is superior to ALT, which is the standard biomarker for necroinflammatory activity 15 .
ActiTest and FibroTest make it possible to identify asymptomatic carriers of hepatitis B 15 .
ActiTest and FibroTest make it possible to identify potential treatments and monitor the progression of chronic viral hepatitis 9 .
ActiTest is a quantitative biomarker which has been validated in subjects who are at high metabolic risk, whether or not this is accompanied by severe obesity 16 7 .
SteatoTest 2 estimates the degree of steatosis in subjects at high metabolic risk, patients who consume excess alcohol or chronic carriers of the hepatitis B or C viruses. 17 1 18 19 11 20 7
As a quantitative biomarker for steatosis, SteatoTest 2 allows longitudinal monitoring to be performed on patients. 13 14
SteatoTest 2 is approved as a predictor of cardiovascular risk associated with steatosis 21 .
SteatoTest 2 is equivalent to SteatoTest (non-inferiority) but more convenient (no BMI, no bilirubin) 22 .
NashTest 2 estimates the liver inflammation as a quantitative assessment 23 of steatohepatitis and the prediction of liver outcome 24 .
NashTest 2 is constructed using updated histological consensus on NASH definition and meets the requirements of the new SAF scoring system 25 7 .
It is a quantitative test, assessing the severity of the liver inflammation (NASH), without the need of BMI.
NashTest 2, when combined with FibroTest, has demonstrated its value in screening for NASH in patients with metabolic risk factors 26 27 28 .
AshTest 29 is a fast alternative to transjugular hepatic biopsies, which therefore makes it possible to treat acute alcoholic steatohepatitis (ASH) in patients suffering from alcohol-related liver disease 30 18 29 19 .
NASH-FibroTest combines ten standard biomarkers:
These markers are weighted depending on the patient's age, sex.
NASH-FibroTest tests must be done on an empty stomach in any local medical test laboratory that complies with BioPredictive's technical recommendations.
There is NO NEED for patient's BMI, nor its weight or height.
Where to do the test Technical RecommendationsYou can also access all the scientific literature about NASH-FibroTest, and the latest news on non-invasive testing for the liver.