BioPredictive's five main diagnostic tests are included in NASH-FibroTest, for a complete assessment of the condition of the liver and the five main causes of liver disease 1 .
NASH-FibroTest = FibroTest + SteatoTest 2 + NashTest 2 + ActiTest + AshTest
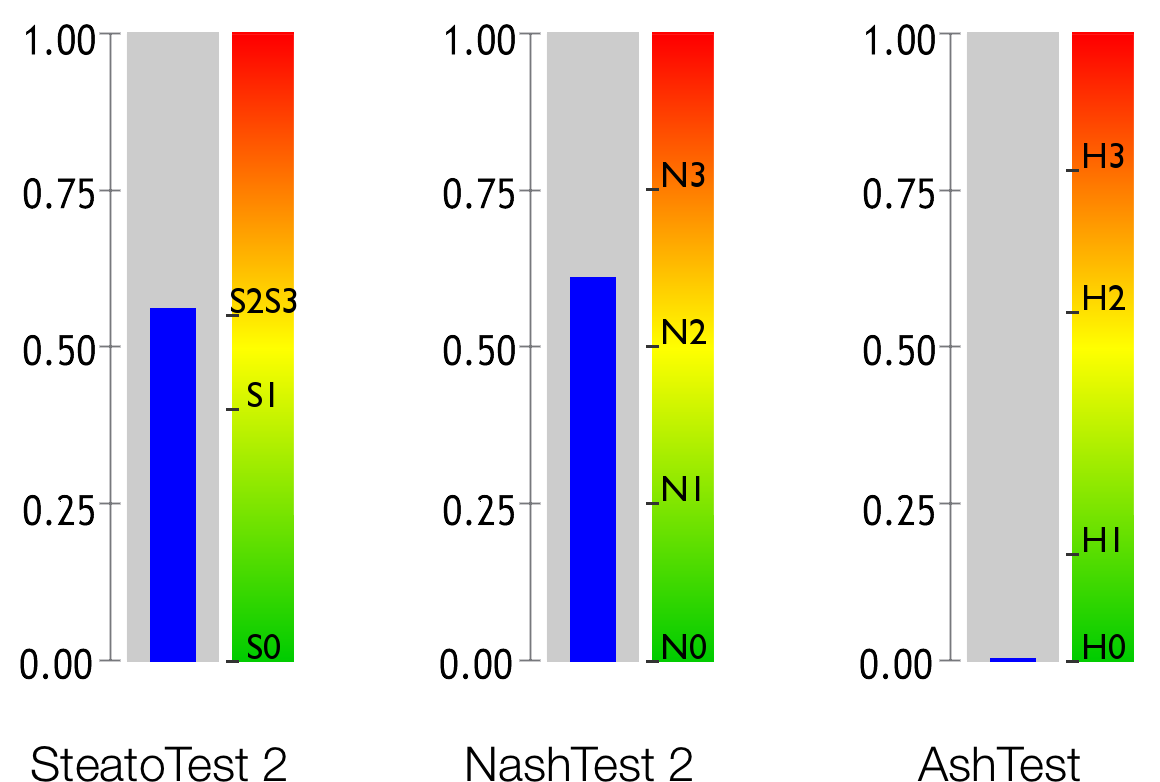
Hepatic steatosis, which is assessed by the SteatoTest 2, is a build-up of fat in the liver, which frequently causes elevated levels of Gamma-GT and transaminases.
Non-alcoholic steatohepatitis (NASH) is an inflammatory disease of the liver which is caused by metabolic conditions including excess weight, arterial hypertension (high blood pressure) and abnormal levels of triglycerides or cholesterol. The NashTest 2 evaluates the level of necroinflammatory activity caused by the metabolic condition.
Steatohepatita alcoolică (ASH) este o boală inflamatorie a ficatului cauzată de consumul excesiv de alcool. Testul AshTest evaluează nivelul acestei activități necroinflamatorii cauzate de alcool.
Fibroza și activitatea necroinflamatorie sunt principalele două cauze ale hepatopatiei.
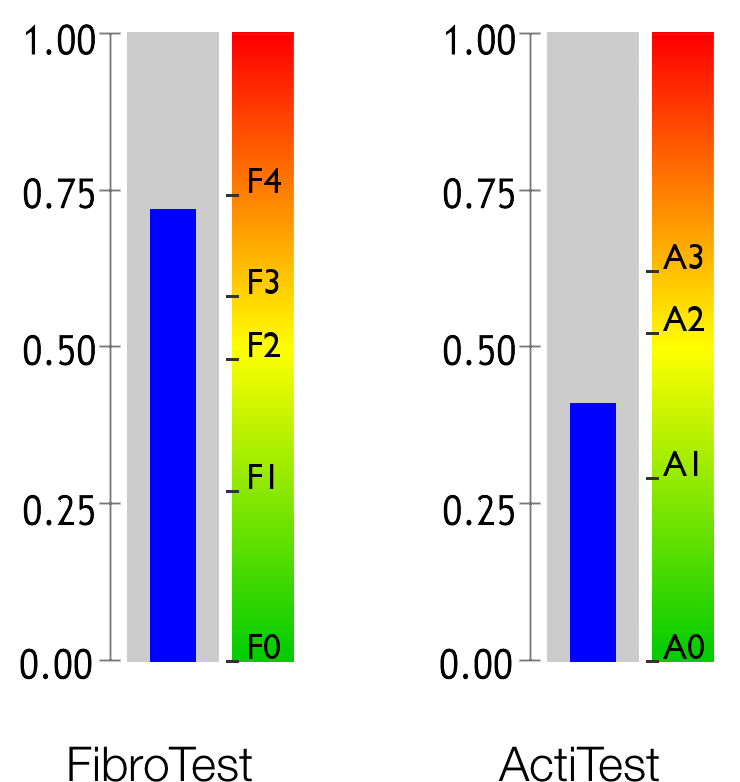
Fibroza este o afecțiune medicală cauzată de reacția ficatului bolnav. Fibroza hepatică este comparată de obicei cu o formă de țesut de cicatrizare care avansează pe suprafața ficatului. Cea mai gravă formă de fibroză este cunoscută drept ciroză.
Activitatea se referă la nivelul de inflamație a ficatului cauzată de boală. Adesea este comparată cu o arsură.
FibroTest este recomandat de WHO - Asociația Mondială a Sănătății 2 , Asociația Americană pentru Studiul Hepatopatiilor (AASLD) 3 , Asociația Europeană pentru Studiul Ficatului (EASL) 4 și Asociația Asia-Pacific pentru Studiul Ficatului (APASL) 5 pentru testarea fibrozei hepatice la pacienții cu hepatită C cronică, cu sau fără co-infectări cu HIV, cât și pentru pacienții cu afecțiuni metabolice sau care consumă alcool în exces. 6 7 8
FibroTest is used to give access to non-interferon treatments for combating the hepatitis C virus and for patient monitoring 9 .
Atunci când este combinat cu ActiTest, testul FibroTest face posibilă identificarea purtătorilor asimptomatici ai virusului hepatic B, dar și a posibilelor tratamente 10 .
FibroTest este conceput special pentru ciroză și este aprobat pentru clasificarea gradului de gravitate a bolii în una din cele trei clase 11 .
FibroTest este singurul test capabil să clasifice stadiile incipiente ale fibrozei 12 .
FibroTest poate fi utilizat pentru monitorizarea longitudinală a pacienților cu hepatopatie cronică 9 13 14 .
Testul ActiTest este superior ALT-ului, care este biomarkerul standard pentru activitatea necroinflamatorie 15 .
ActiTest și FibroTest fac posibilă identificarea purtătorilor asimptomatici de hepatită B 15 .
ActiTest și FibroTest fac posibilă identificarea posibilelor tratamente și monitorizarea progresului hepatitei virale cronice 9 .
ActiTest este un biomarker cantitativ care a fost validat la subiecții cu un risc metabolic ridicat, însoțit sau nu de obezitate severă 16 7 .
SteatoTest 2 estimates the degree of steatosis in subjects at high metabolic risk, patients who consume excess alcohol or chronic carriers of the hepatitis B or C viruses. 17 1 18 19 11 20 7
As a quantitative biomarker for steatosis, SteatoTest 2 allows longitudinal monitoring to be performed on patients. 13 14
SteatoTest 2 is approved as a predictor of cardiovascular risk associated with steatosis 21 .
SteatoTest 2 is equivalent to SteatoTest (non-inferiority) but more convenient (no BMI, no bilirubin) 22 .
NashTest 2 estimates the liver inflammation as a quantitative assessment 23 of steatohepatitis and the prediction of liver outcome 24 .
NashTest 2 is constructed using updated histological consensus on NASH definition and meets the requirements of the new SAF scoring system 25 7 .
It is a quantitative test, assessing the severity of the liver inflammation (NASH), without the need of BMI.
NashTest 2, when combined with FibroTest, has demonstrated its value in screening for NASH in patients with metabolic risk factors 26 27 28 .
AshTest 29 este o alternativă rapidă la biopsiile hepatice transjugulare, care face așadar posibilă tratarea steatohepatitei alcoolice acute (ASH) la pacienții care suferă de hepatopatii provocate de consumul de alcool 30 18 29 19 .
NASH-FibroTest combines ten standard biomarkers:
These markers are weighted depending on the patient's age, sex.
NASH-FibroTest tests must be done on an empty stomach in any local medical test laboratory that complies with BioPredictive's technical recommendations.
There is NO NEED for patient's BMI, nor its weight or height.
Unde se poate face testul Recomandări tehniceYou can also access all the scientific literature about NASH-FibroTest, and the latest news on non-invasive testing for the liver.